Salicylic acid
| Salicylic acid | |
|---|---|
 |
 |
|
2-Hydroxybenzoic acid
|
|
| Identifiers | |
| CAS number | 69-72-7 |
| PubChem | 338 |
| ChemSpider | 331 |
| EC number | 200-712-3 |
|
SMILES
OC(=O)c1ccccc1O
|
|
| Properties | |
| Molecular formula | C7H6O3 |
| Molar mass | 138.12 g mol−1 |
| Density | 1.443 g/cm3 |
| Melting point |
159.0 °C, 432 K, 318 °F |
| Boiling point |
211 °C (20 mmHg) |
| Solubility in water | 0.2 g/100 mL H2O (20 °C) |
| Solubility in chloroform, ethanol, methanol | Chloroform 0.19 M, ethanol 1.84 M, methanol 2.65 M [1] |
| Related compounds | |
| Related compounds | Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid |
| Hazards | |
| MSDS | Oxford MSDS |
| EU Index | 200-712-3 |
| EU classification | Harmful (Xn) |
| R-phrases | R22 R36 R38 R61 |
| S-phrases | S22 S26 S36 S37 S39 |
| NFPA 704 |
 1
2
0
|
| Flash point | 157 °C |
| Autoignition temperature |
545 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Salicylic acid (from Latin salix, willow tree, from the bark of which the substance is obtained) is a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being a compound that is chemically similar to but not identical to the active component of aspirin (acetylsalicylic acid), it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.
Contents |
Chemistry
Salicylic acid has the formula C6H4(OH)COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzenecarboxcylic acid. It is poorly soluble in water (0.2 g/100 ml H2O at 20 °C).[2] Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid.
Plant hormone
Salicylic acid (SA) is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake and transport. SA also induces specific changes in leaf anatomy and chloroplast structure. SA is involved in endogenous signaling, mediating in plant defense against pathogens.[3] It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related proteins.[4] It is involved in the systemic acquired resistance (SAR) in which a pathogenic attack on one part of the plant induces resistance in other parts. The signal can also move to nearby plants by salicyclic acid being converted to the volatile ester, methyl salicylate.[5]
Production
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine-independent pathway.
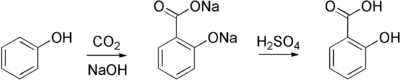
Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid:
It can also be prepared by the hydrolysis of Aspirin (acetylsalicylic acid)[6] or methyl salicylate (Oil of Wintergreen) with a strong acid or base.
History
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy was also mentioned in texts from ancient Sumer, Lebanon, and Assyria. The Cherokee and other Native Americans used an infusion of the bark for fever and other medicinal purposes for centuries.[7] The medicinal part of the plant is the inner bark and was used as a pain reliever for a variety of ailments. The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[8]
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. A larger amount of the substance was isolated by in 1828 by Henri Leroux a French pharmacist. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.[9]
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
Medicinal and cosmetic uses
Salicylic acid is known for its ability to ease aches and pains and reduce fevers. These medicinal properties, particularly fever relief, have been known since ancient times, and it was used as an anti-inflammatory drug.[10]
In modern medicine, salicylic acid and its derivatives are used as constituents of some rubefacient products. For example, methyl salicylate is used as a liniment to soothe joint and muscle pain, and choline salicylate is used topically to relieve the pain of aphthous ulcers.

As with other beta hydroxy acids, salicylic acid is a key ingredient in many skin-care products for the treatment of acne, psoriasis, calluses, corns, keratosis pilaris, and warts.[11] It works as both a keratolytic and comedolytic agent by causing the cells of the epidermis to shed more readily, opening clogged pores and neutralizing bacteria within, preventing pores from clogging up again by constricting pore diameter, and allowing room for new cell growth.[12] Because of its effect on skin cells, salicylic acid is used in several shampoos used to treat dandruff. Use of concentrated solutions of salicylic acid may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock.[13][14]
Bismuth subsalicylate, a salt of bismuth and salicyclic acid, is the active ingredient in stomach relief aids such as Pepto-Bismol. Bismuth subsalicylate helps control nausea, heartburn, indigestion, upset stomach, and diarrhea. It is also a very mild antibiotic.
Other uses
- Although toxic in large quantities, salicylic acid is used as a food preservative. For some people with salicylate sensitivity even these small doses can be harmful.
- Sodium salicylate is a useful phosphor in the vacuum ultraviolet with nearly flat quantum efficiency for wavelengths between 10 to 100 nm.[15] It fluoresces in the blue at 420 nm. It is easily prepared on a clean surface by spraying a saturated solution of the salt in methanol followed by evaporation.
Safety
Salicylic acid has an ototoxic effect by inhibiting prestin.[16] It can induce transient hearing loss in zinc-deficient individuals.
This finding is based on clinical studies with rats. An injection of salicylic acid induced hearing loss in zinc-deficient rats, while a simultaneous injection of zinc reversed the hearing loss. An injection of magnesium in the zinc-deficient rats did not reverse the salicylic acid-induced hearing loss.
Salicylic acid is used to treat acne, warts and other dermatological problems. There are no studies specifically looking at topical salicylic acid in pregnancy. Oral salicylic acid has not been associated with an increase in malformations if used during the first trimester, but use in late pregnancy has been associated with bleeding, especially intracranial bleeding.[17] The risks of aspirin late in pregnancy are probably not relevant for a topical exposure to salicylic acid, even late in the pregnancy, because of its low systemic levels. Topical salicylic acid is common in many over-the-counter dermatological agents, and the lack of adverse reports suggests a low teratogenic potential.[18]
Salicylic acid overdose can lead to salicylate intoxication, which often presents clinically in a state of metabolic acidosis with compensatory respiratory alkalosis. In patients presenting with an acute overdose, a 16% morbidity rate and a 1% mortality rate are observed.[19]
Some people are hypersensitive to salicylic acid and related compounds.
The United States Food and Drug Administration recommends the use of sun protection when using skincare products containing salicylic acid (or any other BHA) on sun-exposed skin areas.[20]
There is data to support an association between exposure to salicylic acid and Reye's Syndrome. The National Reye's Syndrome Foundation cautions against the usage of these substances, and other substances similar to aspirin, on children and adolescents.
Epidemiological research has shown an association between the development of Reye's Syndrome and the use of aspirin (a salicylate compound) for treating the symptoms of influenza-like illnesses, chicken pox, colds, etc.
The U.S. Surgeon General, the Food and Drug Administration, the Centers for Disease Control and Prevention, and the American Academy of Pediatrics recommend that aspirin and combination products containing aspirin not be given to children under 19 years of age during episodes of fever-causing illnesses.[21]
See also
- Trolamine salicylate
Footnotes
- ↑ Solubility of salicylic acid in non-aqueous solvents
- ↑ "Salicilyc acid". http://www.inchem.org/documents/icsc/icsc/eics0563.htm. Retrieved 2008-10-13.
- ↑ S. Hayat, A. Ahmad (2007). Salicylic acid - A Plant Hormone. Springer. ISBN 1402051832.
- ↑ HOOFT VAN HUIJSDUIJNEN. Induction by Salicylic Acid of Pathogenesis-related Proteins and Resistance to Alfalfa Mosaic Virus Infection in Various Plant Species. http://vir.sgmjournals.org/cgi/reprint/67/10/2135.pdf. Retrieved 2009-05-28.
- ↑ Plant Physiology Third Edition, Taiz and Zeiger, 2002, page 306
- ↑ "Hydrolysis of ASA to SA". http://www.crscientific.com/article-aspirin.html. Retrieved July 31, 2007.
- ↑ Paul B. Hemel and Mary U. Chiltoskey, Cherokee Plants and Their Uses -- A 400 Year History, Sylva, NC: Herald Publishing Co. (1975); cited in Dan Moerman, A Database of Foods, Drugs, Dyes and Fibers of Native American Peoples, Derived from Plants.[1] A search of this database for "salix AND medicine" finds 63 entries.
- ↑ Stone, E (1763). "An Account of the Success of the Bark of the Willow in the Cure of Agues". Philosophical Transactions 53: 195–200. doi:10.1098/rstl.1763.0033. http://www.journals.royalsoc.ac.uk/openurl.asp?genre=article&issn=0260-7085&volume=53&spage=195.
- ↑ Diarmuid Jeffreys. (2005). Aspirin : the remarkable story of a wonder drug. New York, NY: Bloomsbury. pp. 38–40. ISBN 9781582346007. http://books.google.de/books?id=x-sL4iNQOgcC&pg=PA38.
- ↑ Philip A. Mackowiak (2000). "Brief History of Antipyretic Therapy". Clinical Infectious Diseases, 31: 154–156. doi:10.1086/317510.
- ↑ http://www3.interscience.wiley.com/journal/119449119/abstract?CRETRY=1&SRETRY=0
- ↑ Salicylic Acid Essential Actives, KAVI.
- ↑ Grimes P.E. (1999). "The Safety and Efficacy of Salicylic Acid Chemical Peels in Darker Racial-ethnic Groups". Dermatologic Surgery 25 (1): 18–22. doi:10.1046/j.1524-4725.1999.08145.x. PMID 9935087.
- ↑ Roberts W. E. (2004). "Chemical peeling in ethnic/dark skin". Dermatologic Therapy 17 (2): 196. doi:10.1111/j.1396-0296.2004.04020.x. PMID 15113287.
- ↑ JAR Samson Techniques of Vacuum Ultraviolet Spectroscopy
- ↑ Wecker, H.; Laubert, A. (2004). "Reversible hearing loss in acute salicylate intoxication" (in German). HNO 52 (4): 347–51. doi:10.1007/s00106-004-1065-5. PMID 15143764.
- ↑ Rumack, Cm; Guggenheim, Ma; Rumack, Bh; Peterson, Rg; Johnson, Ml; Braithwaite, Wr (1981). "Neonatal intracranial hemorrhage and maternal use of aspirin.". Obstetrics and gynecology 58 (5 Suppl): 52S–6S. ISSN 0029-7844. PMID 7312229.
- ↑ Acne and Pregnancy
- ↑ http://emedicine.medscape.com/article/818242-overview
- ↑ "Beta Hydroxy Acids in Cosmetics". http://www.cfsan.fda.gov/~dms/cos-bha.html. Retrieved 2007-11-23.
- ↑ "Asprin / Salicylates and Reye's Syndrome". http://www.reyessyndrome.org/aspirin.html. Retrieved 2009-05-22.
External links
|
||||||||||||||||||||||||||||||||
|
|||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||
|
|||||